To minimize the financial and administrative burden on patients who are seeking single-patient expanded access (EA) of investigational products, Western Institutional Review Board (WIRB) provides IRB review of expanded access applications at no cost, when there is no local institutional review board to provide this review.
WIRB provides this review in accordance with the existing FDA regulations for expanded access, and accepts the FDA form 3926 as the IRB application for review. Responses to the application are generally provided within 24-48 hours after submission of the application in non-emergency requests, unless information is missing.
In 2018 WIRB approved 100% of the single-patient EA requests that were submitted for review. Nine percent of the requests were for patients under the age of 18.
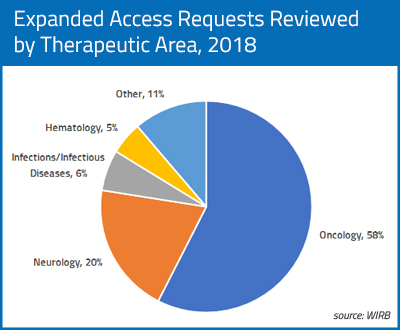
In 2018, 58% of the single-patient EA applications for which WIRB provided review were for oncology or oncology-related indications, including multiple applications for patients with breast cancer, brain cancer, prostate cancer, acute leukemia, lung cancer, colorectal carcinoma, pancreatic adenocarcinoma, bladder cancer, appendiceal carcinoma, and urothelial carcinoma. Applications were also received for single patients with less common cancers such as cholangiosarcoma, multiple myeloma and endometrial carcinoma, among others. Applications were also received for products to treat cancer-related indications such as cachexia (inability to eat) in a patient with lung cancer.
The next most frequent therapeutic area for single-patient IND requests was neurology, with multiple requests for patients with ALS, progressive supranuclear palsy, Alzheimer’s disease, and refractory cluster headaches and single requests for patient with epileptic conditions, myasthenia gravis, and Parkinson’s disease. The third most common therapeutic area for requests was infections and infectious diseases, with requests for products to treat mycobacterium avium infection, tuberculosis and leprosy. Requests were also received for multiple patients with gastroparesis, major depressive disorder, and amyloidosis.
WIRB remains committed to facilitating the single-patient expanded access process for patients who are using the FDA pathway to obtain investigational products for serious or life-threatening diseases. More information about this program can be found at www.wirb.com, under IRB Review Services.
Don't trust your study to just anyone.
WCG's IRB experts are standing by to handle your study with the utmost urgency and care. Contact us today to find out the WCG difference!